Fresenius Kabi +RFID pre-tagged medications feature a high-performance RFID tag embedded in the label that contains the relevant data that hospitals rely on every day to immediately identify, locate, and manage their inventory. All essential product information is accessible from the tag, which enables an interoperable environment and item-level serialization of each +RFID product.
Manufacturer-prepared Manufacturer-prepared medication and RFID embedded label are prepared under strict FDA guidelines and cGMP processes
Compatible +RFID products are designed for compatibility utilizing GS1 standards for ease of use and peace of mind
Pre-tagged Products arrive pre-tagged and encoded, eliminating tedious manual tagging and associating product information
Efficient Saves time for pharmacy personnel, freeing up specialized resources to focus more on value-added tasks
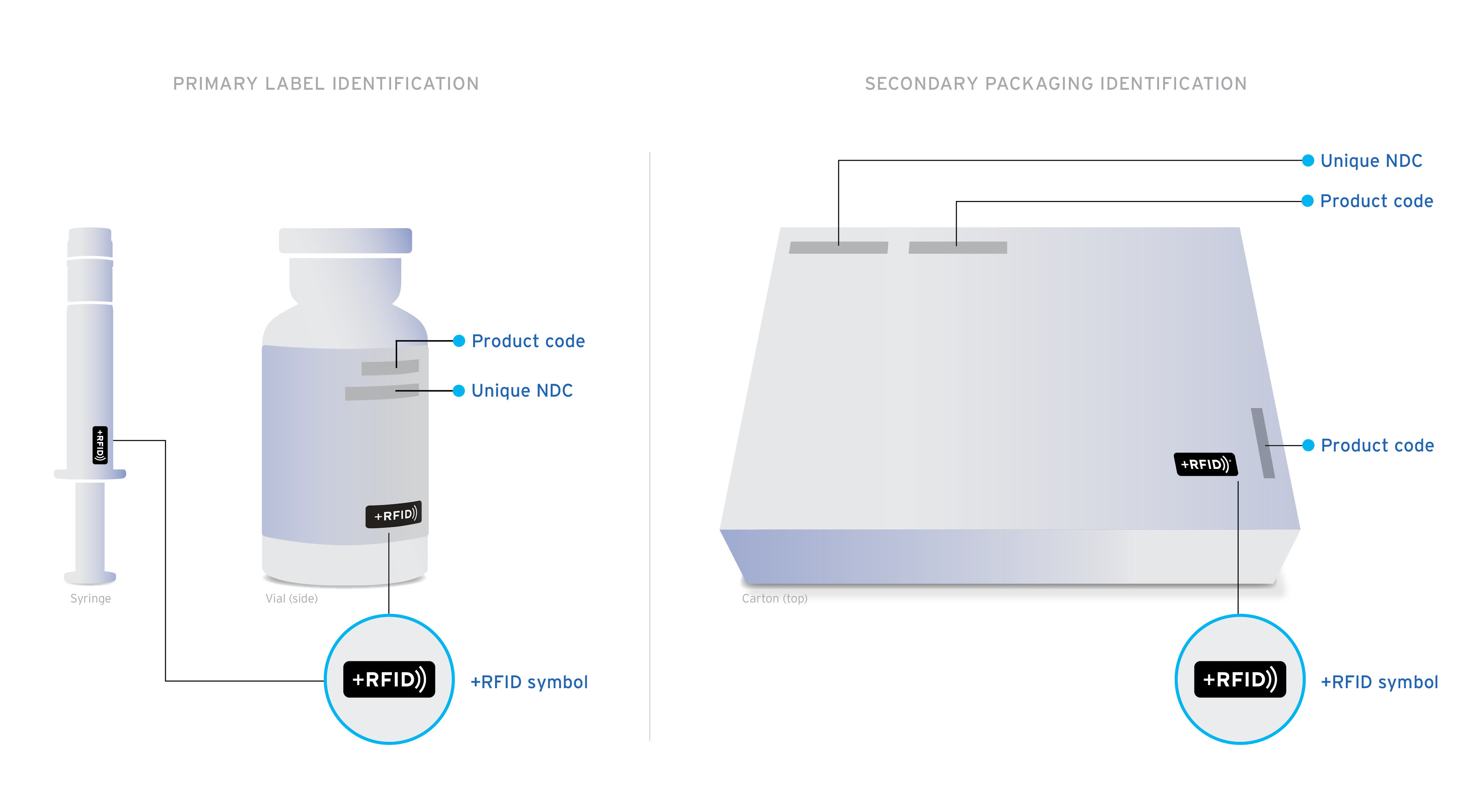
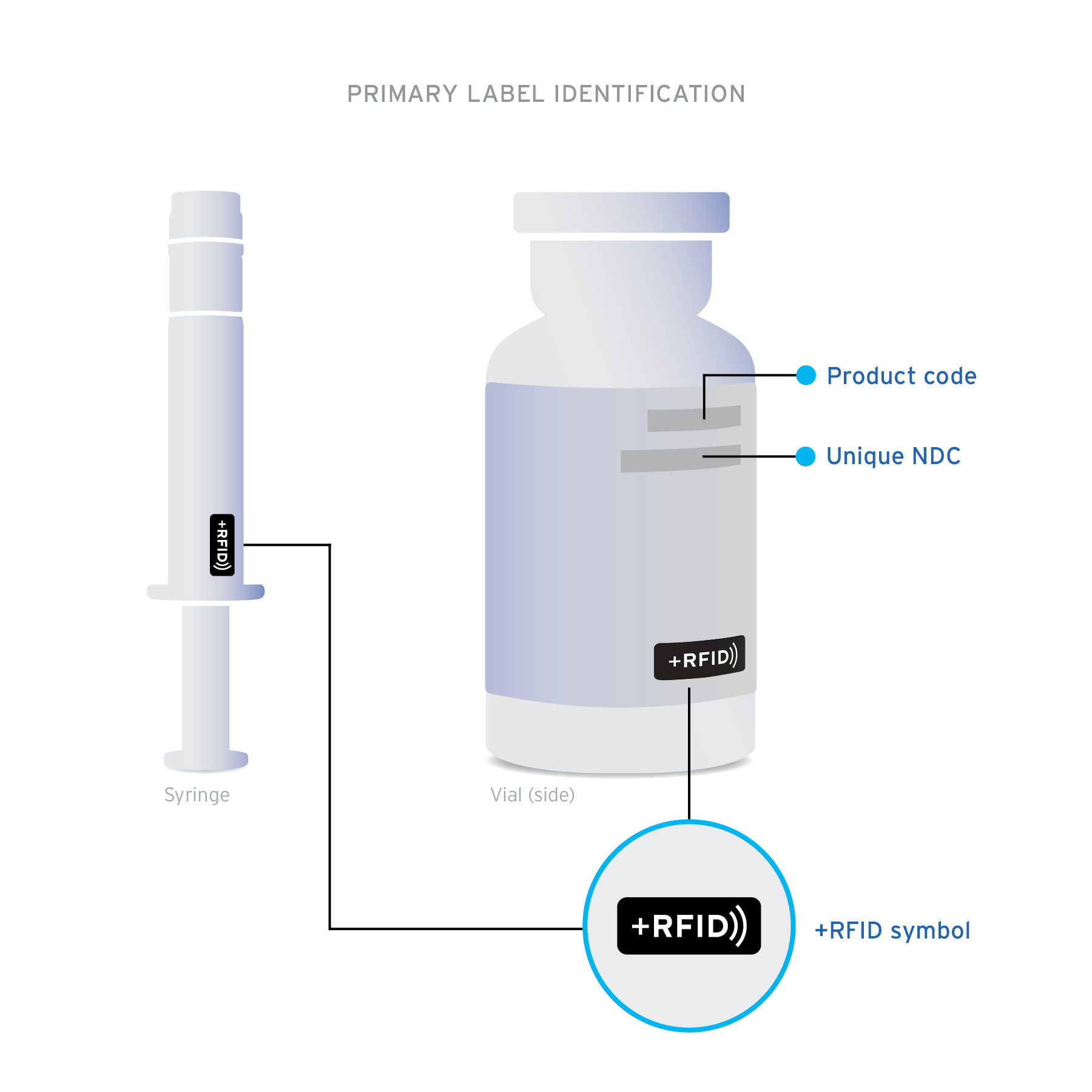
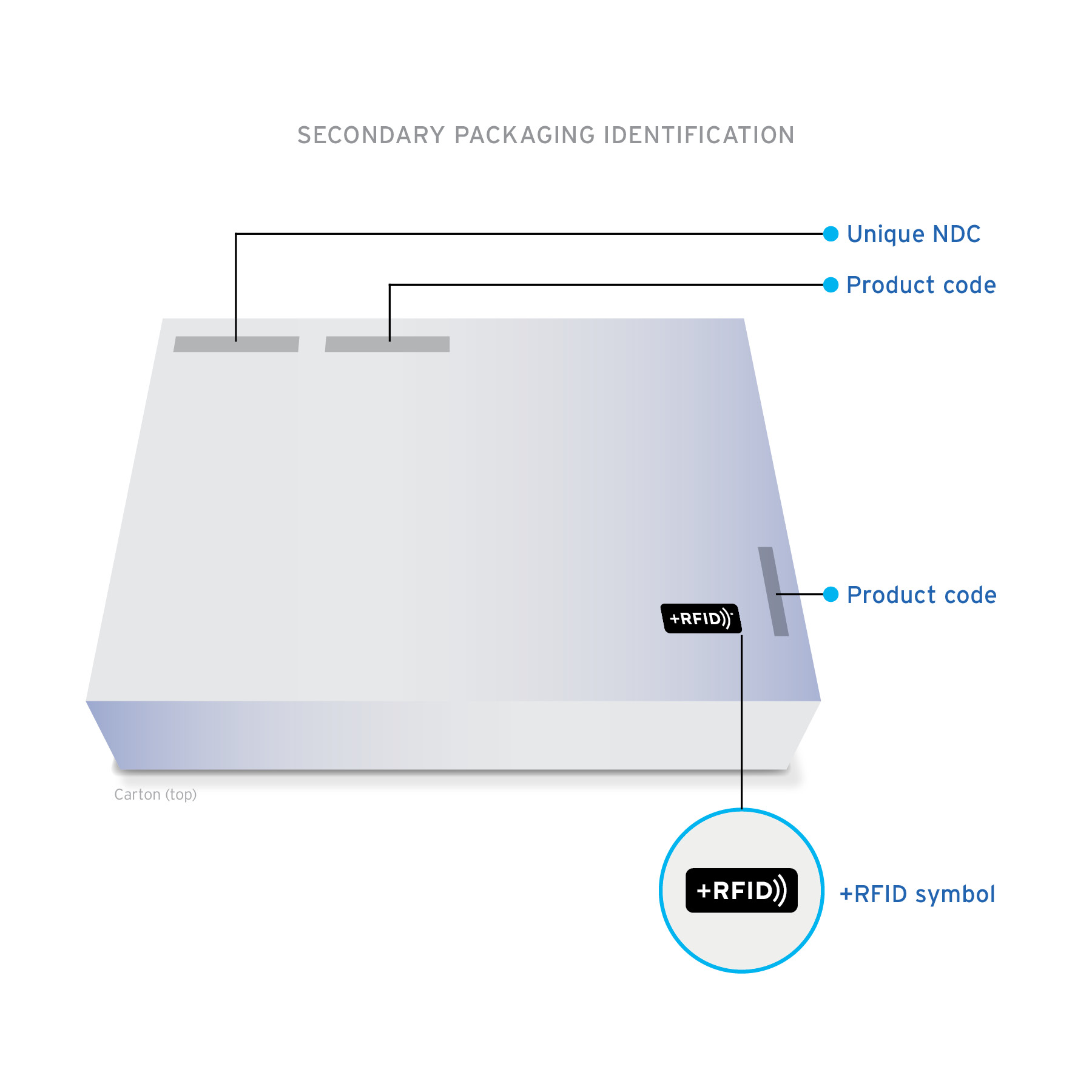
Ready-to-read Custom designed inlays are ready-to-read, loaded with essential drug information: NDC, expiration date, lot, and unique serial number
+RFID pre-tagged medications arrive clearly labeled on product cartons and individual product labels to confirm the presence of RFID-embedded inlays.

Built for the future +RFID pre-tagged medications are built on industry standards to ensure interoperability and actionable data from our tag, enabling multiple read-points to easily leverage information.

GS1 is a global standards organization that brings efficiency and transparency to the supply chain. With guidance from GS1 US, +RFID utilized their traceable serialized numbering standard for each pre-tagged product ensuring compatibility and interoperability with downstream systems.

RAIN RFID promotes the universal adoption of UHF RFID technology. Through RAIN’s partnership, +RFID hopes to create a global-reaching network of technology partners.

ISO sets international standards in a variety of industries. +RFID smart labeled products are developed under ISO/IEC 18000-63:2013, which regulates the RFID technology space.

Axia Lab of Michigan State University (MSU) provides expert led, third-party validation services for RFID tagged pharmaceutical products based on GS1’s Tagged Item Performance Protocol (TIPP) Guidelines to ensure consistent performance and reliability.

Impinj pioneered RAIN RFID, offering RAIN-based products and solutions that deliver critical data about every item that is manufactured, transported and sold. Together with our partners, we help medical providers ensure their assets and inventories are in the right place at the right time.

The purpose of the ARC program is to ensure that RFID tags are able to meet or exceed the levels of performance and quality necessary to provide benefit to the end user in a consistent and cost effective manner. All +RFID inlays are certified by ARC to ensure high quality and performance.
GS1 is a neutral, global collaboration platform that brings industry leaders, government, regulators, academia, and associations together to develop standards-based solutions to address the challenges of data exchange.
Learn more about GS1
+RFID tags are custom designed per medication specification. Each tag provides the highest quality performance and is built to stand up to the demands of the supply chain. View Products page for details on system compatibility.
Your submission has been received.